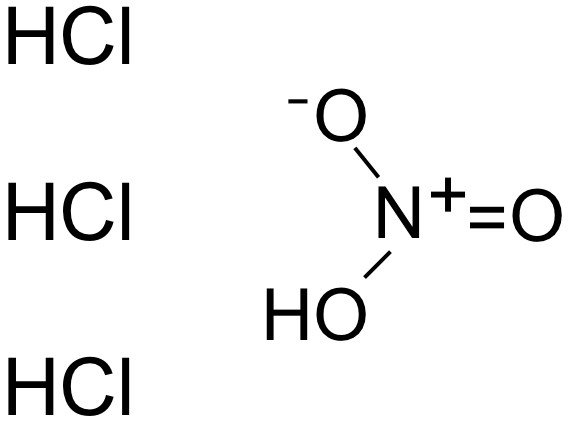
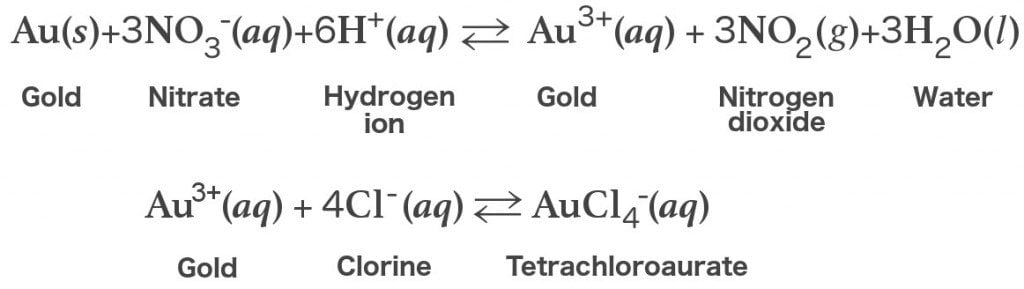SOLVED: Consider the following half-reactions: Pt?+ 2 €" PtCl E? = 1.188 V 2 e" 4CF E? = 0.755 V NO;" 4 H' 3 e NO 2 Ho 0.96 V (a) Write
![What is pK value of the complex [PtCl4] if. Pt^4 + + Cl^ - → [PtCl]^3 + K1 = 10^-2 [PtCl]^3 + + Cl^ - → [PtCl2]^2 + K2 = 10^-3 [PtCl2]^2 + + Cl^ - → [PtCl3]^ + K3 = 10^-4 [PtCl3]^ + + Cl^ - → [PtCl4] K4 = 10^-4 What is pK value of the complex [PtCl4] if. Pt^4 + + Cl^ - → [PtCl]^3 + K1 = 10^-2 [PtCl]^3 + + Cl^ - → [PtCl2]^2 + K2 = 10^-3 [PtCl2]^2 + + Cl^ - → [PtCl3]^ + K3 = 10^-4 [PtCl3]^ + + Cl^ - → [PtCl4] K4 = 10^-4](https://dwes9vv9u0550.cloudfront.net/images/4434478/2fa54aa1-d4bc-4f1e-be65-b6a0fa759e40.jpg)
What is pK value of the complex [PtCl4] if. Pt^4 + + Cl^ - → [PtCl]^3 + K1 = 10^-2 [PtCl]^3 + + Cl^ - → [PtCl2]^2 + K2 = 10^-3 [PtCl2]^2 + + Cl^ - → [PtCl3]^ + K3 = 10^-4 [PtCl3]^ + + Cl^ - → [PtCl4] K4 = 10^-4
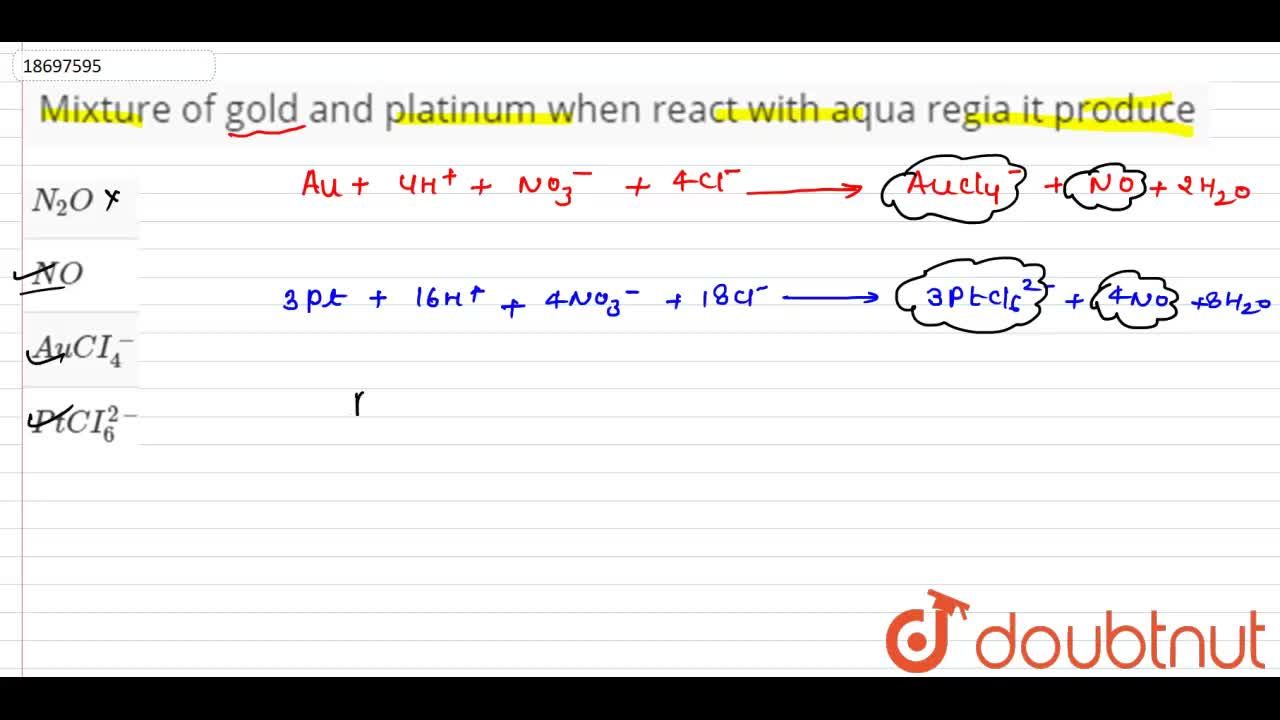
A mixture of gold and platinum when reacts with aqua regia produces which of the following aAuCl4 bPtCl62 cN2O dNO

SOLVED: 0.9 (a) From Table, what are two metals that will not dissolve in MH but will dissolve in M HNO;? (6) Gold, platinum; and iridium not only will not dissolve in
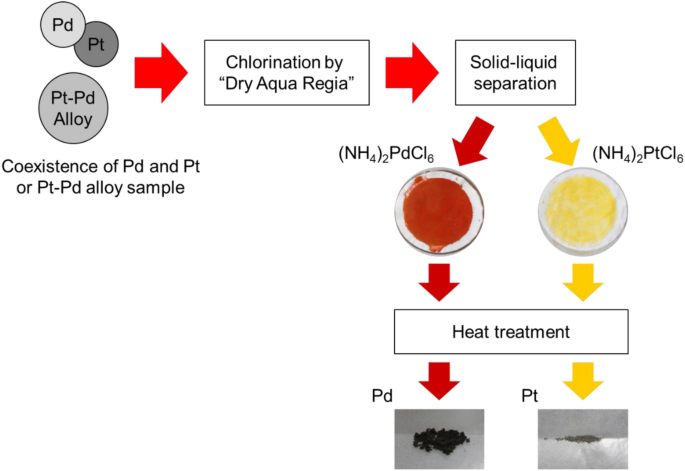
Fundamental Study of Palladium Recycling Using “Dry Aqua Regia” Considering the Recovery from Spent Auto-catalyst | SpringerLink
What is aqua regia? How does it help in dissolving Gold or Platinum. - Sarthaks eConnect | Largest Online Education Community