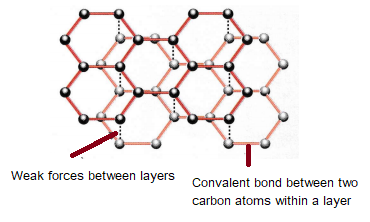
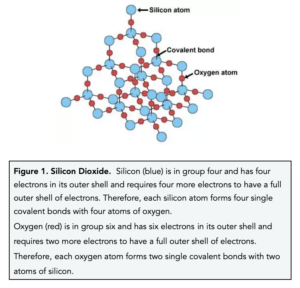
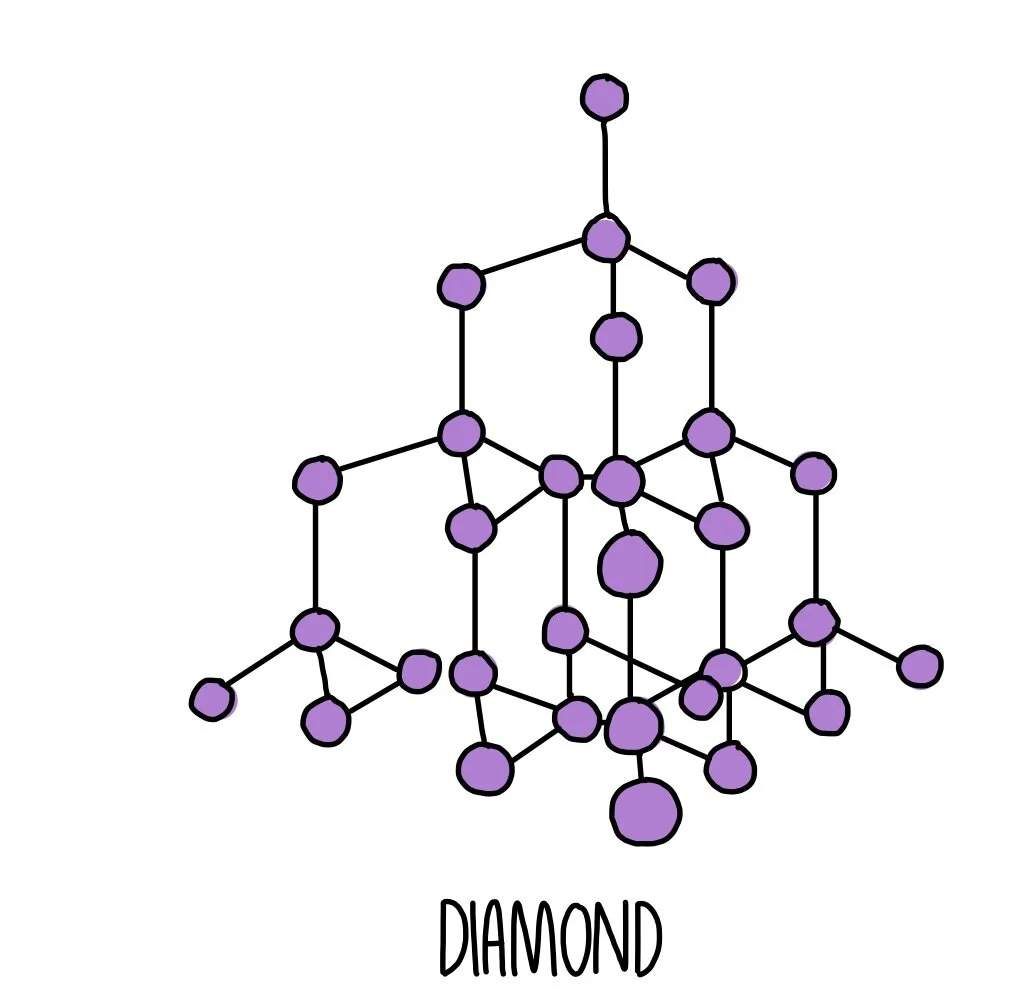
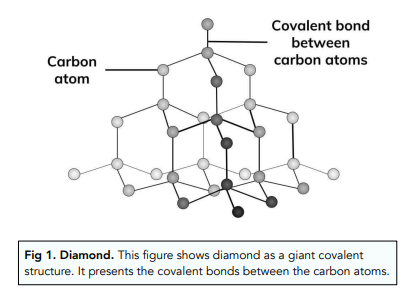
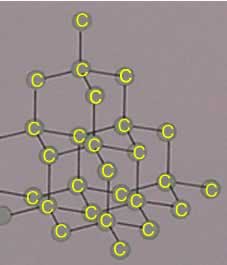
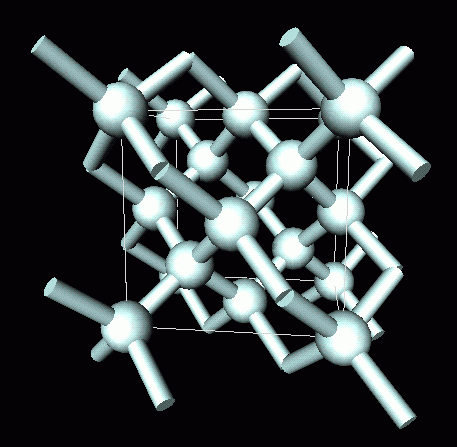
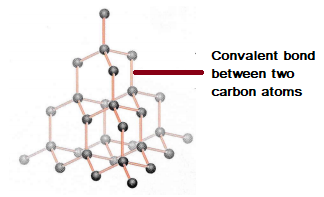
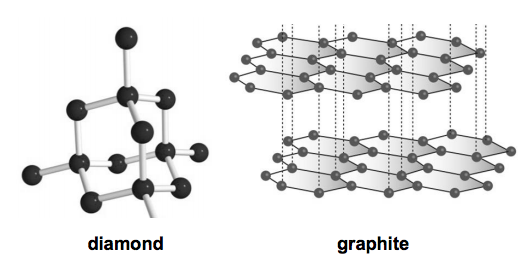
Nami || 15HINee on Twitter: "Let's talk diamonds💎 ✔️Diamonds are the hardest naturally occuring substance on earth. Why? Each carbon atom in diamond is covalently bonded to 4 other carbon atoms. Covalent
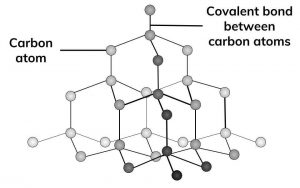
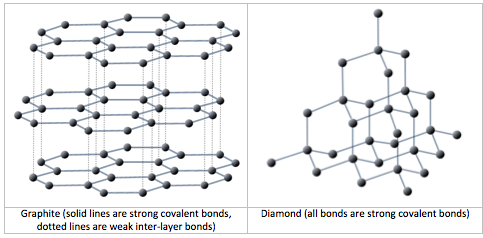
1:50 explain how the structures of diamond, graphite and C60 fullerene influence their physical properties, including electrical conductivity and hardness - TutorMyself Chemistry