
According to law of mass action, for CaCO3(s) CaO (s) + CO2 (g) Rf = rate of forward reaction Rb = rate of backward reactionWhich of the following is true at equilibrium?
CaCO3(s)–> CaO(s) + CO2(g) The process will be spontaneous at (1) All temperatures (2) T>△ H/△ S 3) T

For CaCO3(s) -- CaO(s) +CO2(g) at 977 C , H = 174 KJ / mol Then find - Chemistry - Practical Work - 13011695 | Meritnation.com
![SOLVED: Write the equilibrium constant expression for the following reaction. CaCO3(s) = CaO(s) + CO2(g) Group of answer choices K = [CaO][CO2]/[CaCO3] K = [CO2]/[CaO] K = [CaCO3]/[CaO] K = p(CO2) SOLVED: Write the equilibrium constant expression for the following reaction. CaCO3(s) = CaO(s) + CO2(g) Group of answer choices K = [CaO][CO2]/[CaCO3] K = [CO2]/[CaO] K = [CaCO3]/[CaO] K = p(CO2)](https://cdn.numerade.com/ask_previews/93cce3c7-2535-4756-a8fc-b25c2c056b0a_large.jpg)
SOLVED: Write the equilibrium constant expression for the following reaction. CaCO3(s) = CaO(s) + CO2(g) Group of answer choices K = [CaO][CO2]/[CaCO3] K = [CO2]/[CaO] K = [CaCO3]/[CaO] K = p(CO2)
CaCO3(s) → ← CaO(s) + CO2(g) When heated, calcium carbonate decomposes according to the equation above. In a study of the

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa

OneClass: 4. Calculate ΔGo for the reaction: CaCO3(s) â†' CaO(s) + CO2(g), given ΔGfo CO2(g) = -394...

Question Video: Identifying the Chemical Equation- with State Symbols- That Corresponds to a Chemical Statement | Nagwa

Consider the reaction CaCO3(s) CaO(s) + CO2(g) , in a closed container at equilibrium. What would be the effect of addition of CaCO3(s) on the equilibrium concentration of CO2 ?
CaCO3(s) → ← CaO(s) + CO2(g) When heated, calcium carbonate decomposes according to the equation above. In a study of the

SOLVED: When heated, calcium carbonate decomposes according to the equation: CaCO3 (s) —-> CaO (s) + CO2 (g) A 2.255 g sample of a mixture of CaCO3 and CaO was heated to

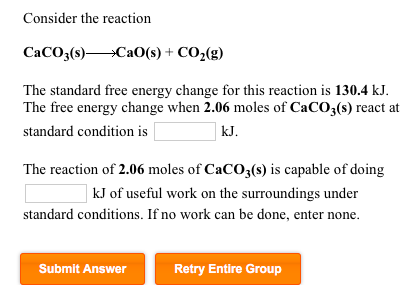


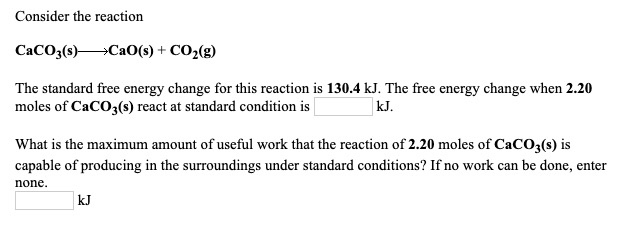
![ANSWERED] W Consider the reaction CaCO3 s CaO s CO ... - Physical Chemistry ANSWERED] W Consider the reaction CaCO3 s CaO s CO ... - Physical Chemistry](https://media.kunduz.com/media/sug-question-candidate/20220425180025492897-4437284.jpg)

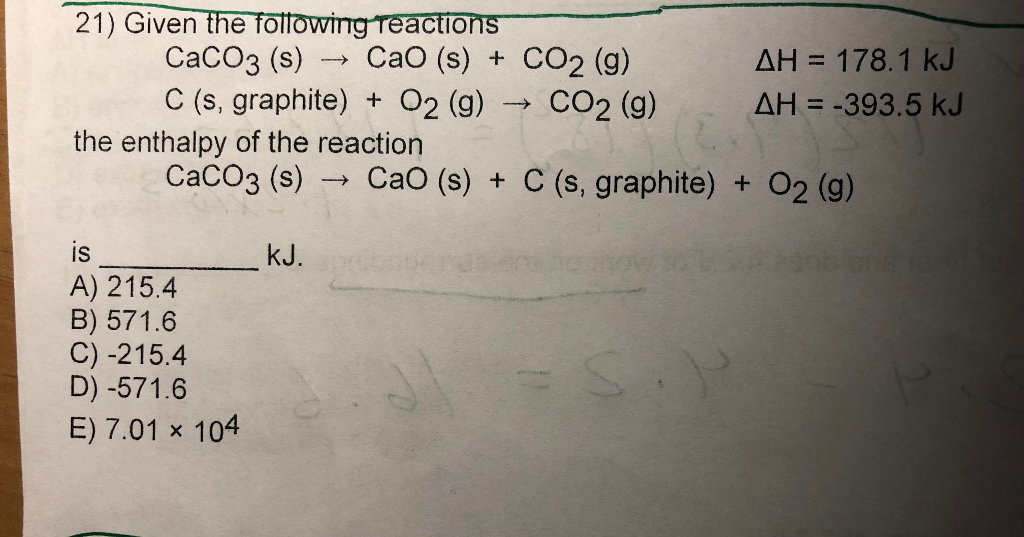

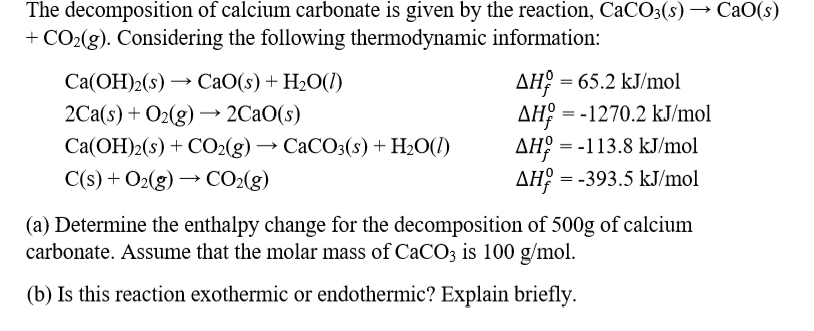
![ANSWERED] CaCO3 s CaO s CO2 g The standard enthalpy... - Physical Chemistry ANSWERED] CaCO3 s CaO s CO2 g The standard enthalpy... - Physical Chemistry](https://media.kunduz.com/media/sug-question-candidate/20220521195736208340-3750862.jpg)