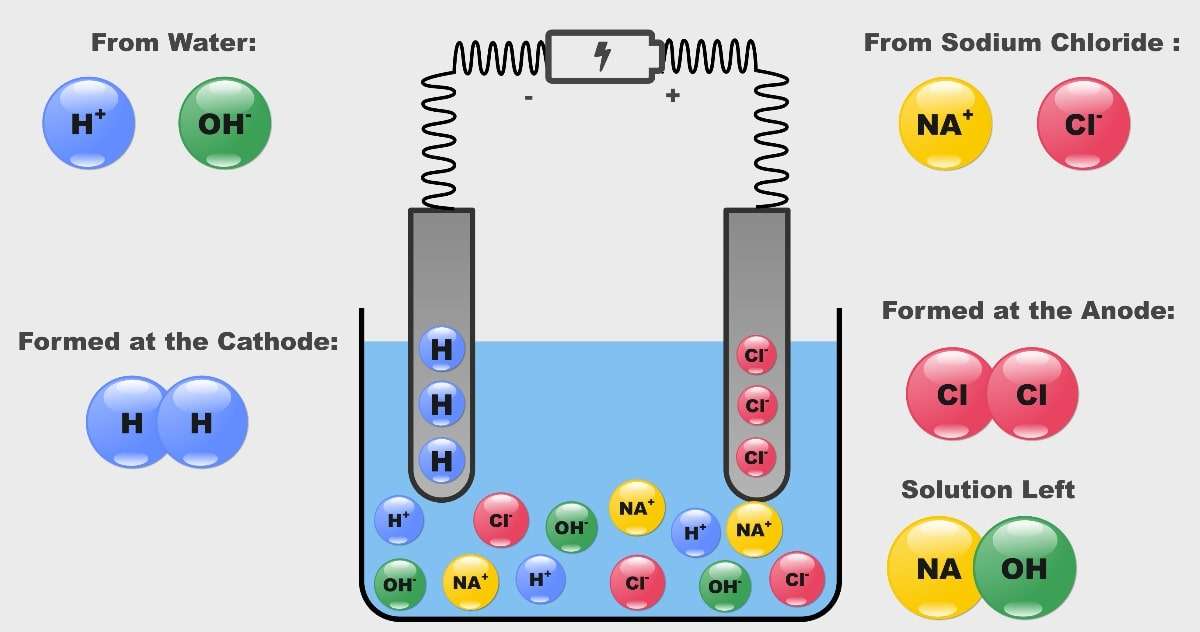
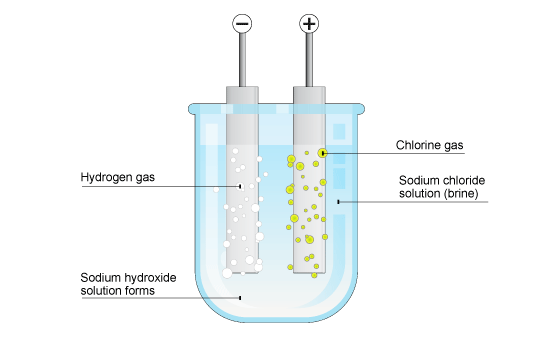
If I add table salt (NaCl) into water and do electrolysis do I get chlorine and hydrogen gases? No oxygen gas at all? - Quora
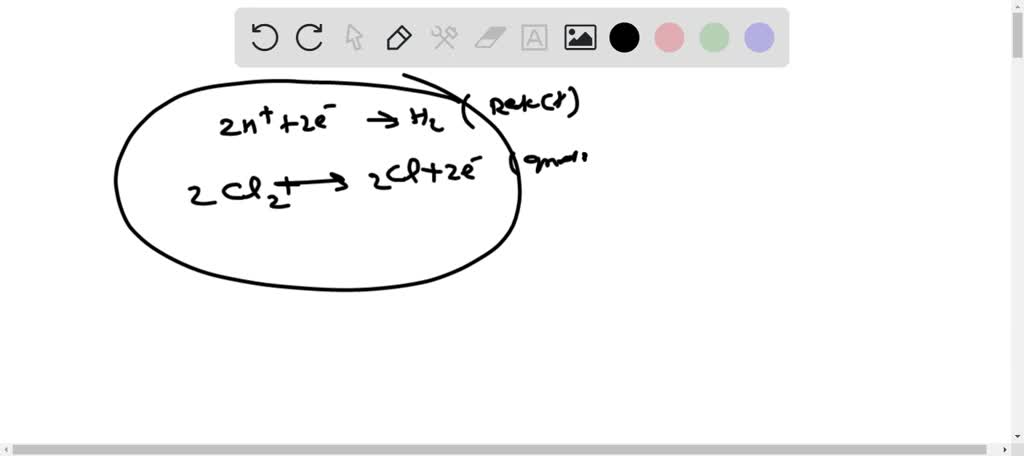
SOLVED: For the electrolysis of sea water to produce hydrogen gas and chlorine gas: a) Write the two half reactions b) Write cell line notation. c) Find standard cell potential.

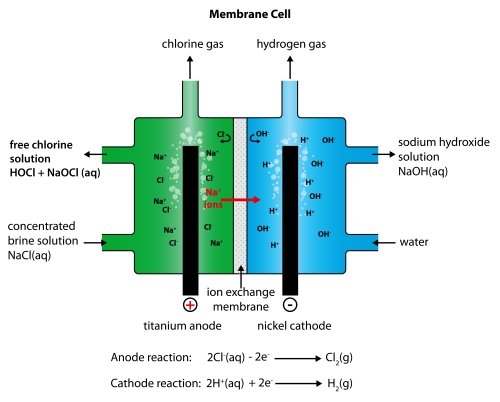
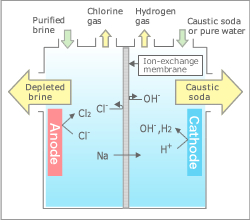
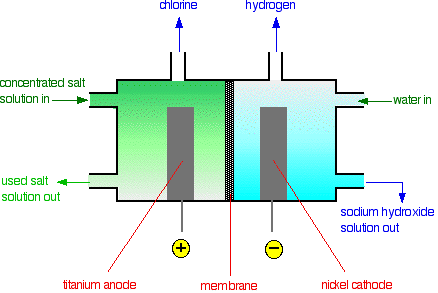
electrolysis of sodium chloride solution brine product equations electrodes anode cathode apparatus electrolyte cell sodium bromide potassium iodide gcse chemistry KS4 science igcse O level revision notes

Seawater electrolysis for simultaneous chlorine and hydrogen production | Particles and Catalysis Research Laboratory

Effects of electrolysis time and electric potential on chlorine generation of electrolyzed deep ocean water - ScienceDirect

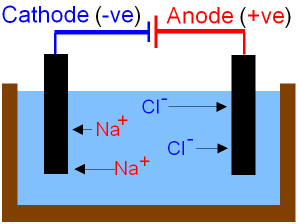
Why is twice as much hydrogen produced than chlorine in the electrolysis of brine? - Chemistry Stack Exchange