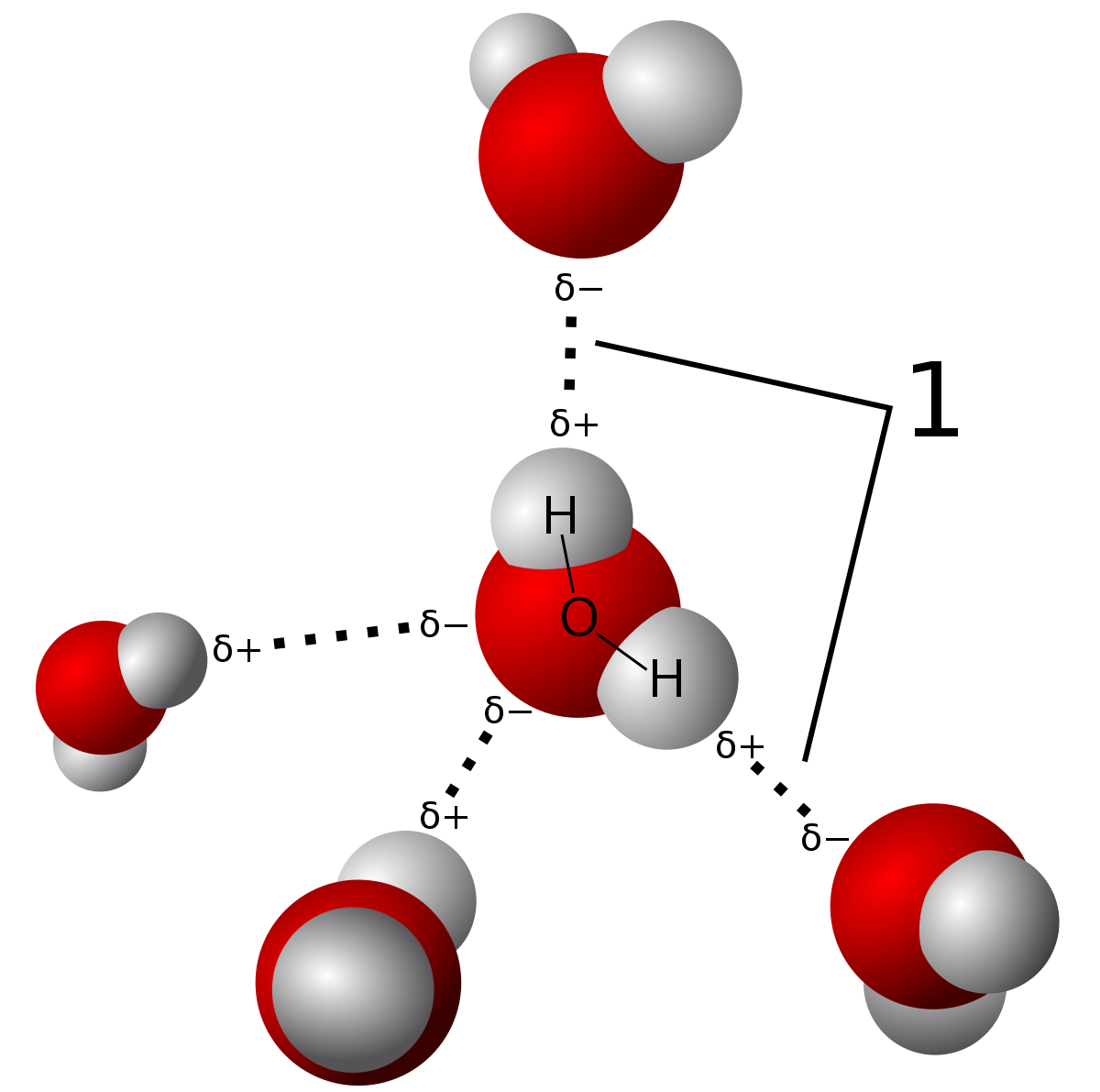
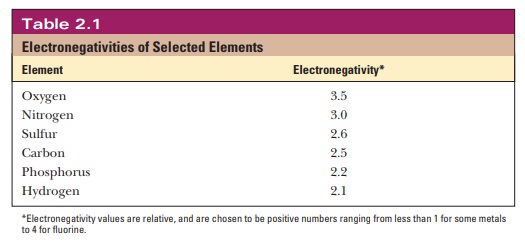
SOLVED: Hydrogen and carbon have comparable electronegativity Hence, covalent bond between and carbon atom hydrogen atom Is generally considered non polar. As shown below; both methane (CH ) and tetrafluoromethane (CF )

ions - Why doesn't 4 hydrogen and 1 carbon form a ionic bond? What is the limit of electrons that can be given away? - Chemistry Stack Exchange
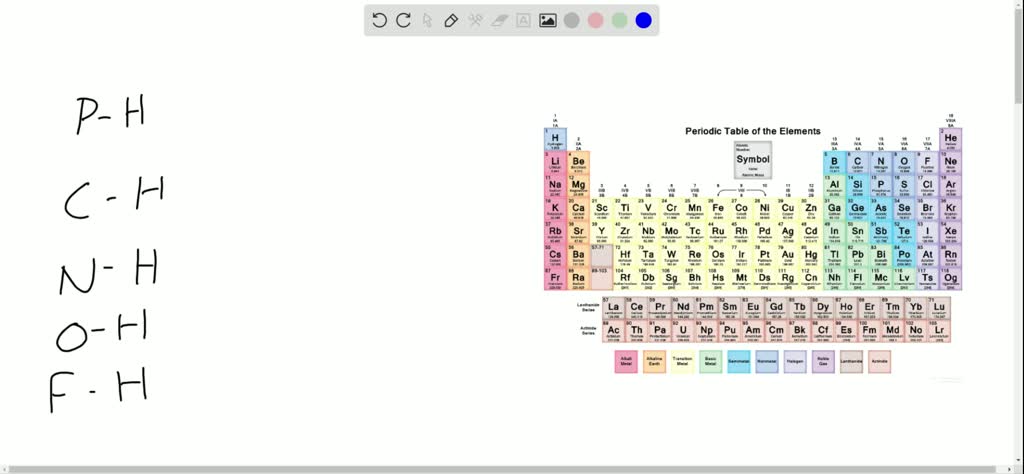
SOLVED:Hydrogen has an electronegativity value between boron and carbon and identical to phosphorus. With this in mind, rank the following bonds in order of decreasing polarity: P-H 𝐎-𝐇, 𝐍-𝐇, 𝐅-𝐇, 𝐂-𝐇
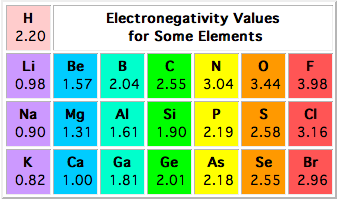
Which one of the following bonds would you expect to be the most polar? a) B–H b) N–H c) P–H d) Al–H e) C–H | Socratic
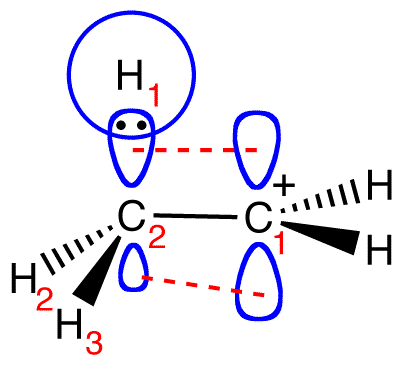
Why do alkyl groups have lower electronegativity versus hydrogen? How does this affect the acidity or alkalinity of alcohols? | Socratic