A level Bond Enthalpy (bond dissociation energy) calculations for Enthalpy of Reaction KS5 GCE chemistry revision notes
66 Enthalpies of formation of co(g),CO2(g),N2O(g)and N2O4(g) are 110, 398, 81, 97 KJ/lol respectively. Find value of H for the reaction. N2O4(g) +3CO(g) — >N20(g)+3CO2(g)

What will be the enthalpy of combustion of carbon to produce carbon monoxide on the basis of data given below? C(s) + O2(g)→ CO2(g) Δ H = - 393.4kJ CO(g) + 1/2O2(g)→

Enthalpy of Solution of Carbon Dioxide in Aqueous Solutions of Monoethanolamine at Temperatures of 322.5 K and 372.9 K and Pressures up to 5 MPa | Journal of Chemical & Engineering Data
The enthalpies of combustion of carbon and carbon monoxide are -393.5 and –283kJ mol^–1 respectively. - Sarthaks eConnect | Largest Online Education Community

Enthalpy of combustion of carbon to CO2 is - 393.5 KJ/mole. The heat released upon the formation of 35.2g of CO2 from carbon and dioxygen gas is.

4.: Phase diagram of carbon dioxide depicted in terms of pressure and... | Download Scientific Diagram

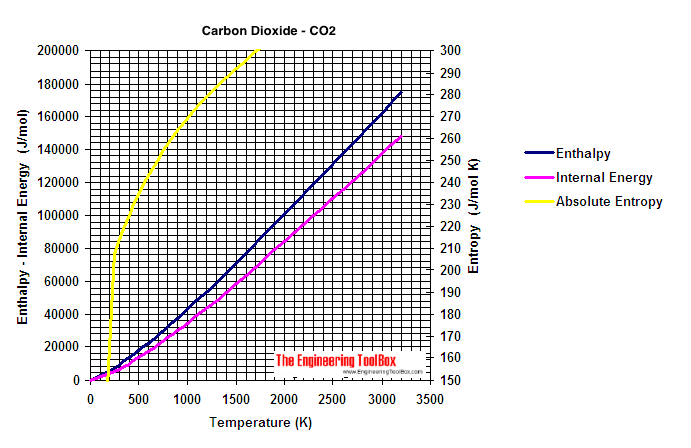
Enthalpy (J/mol) of Carbon Dioxide as a Function of Temperature and Pressure - Carbon Dioxide Thermodynamic Properties Handbook - Wiley Online Library