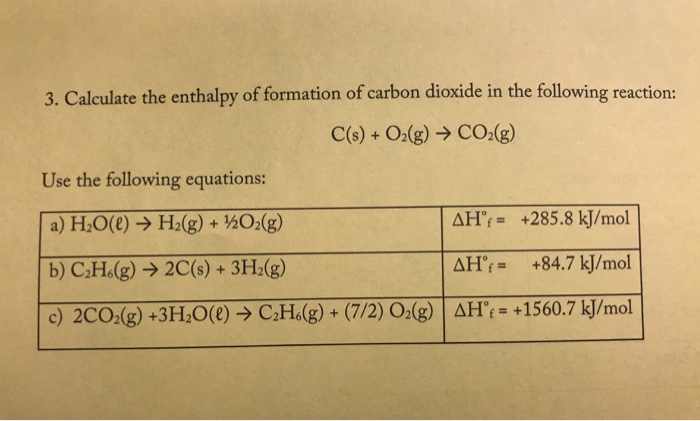
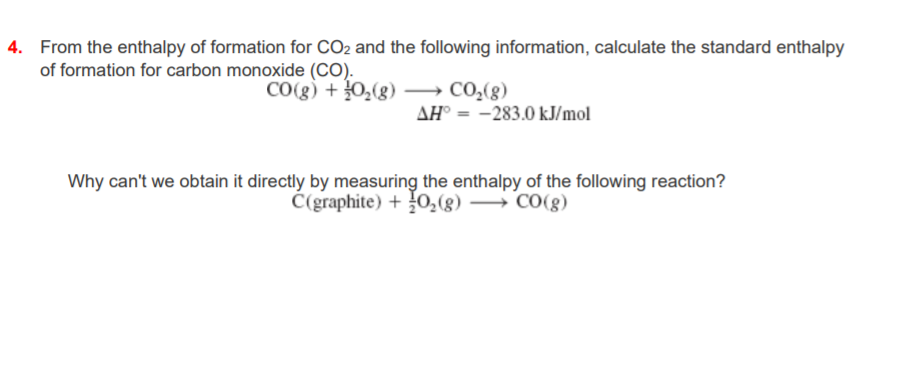
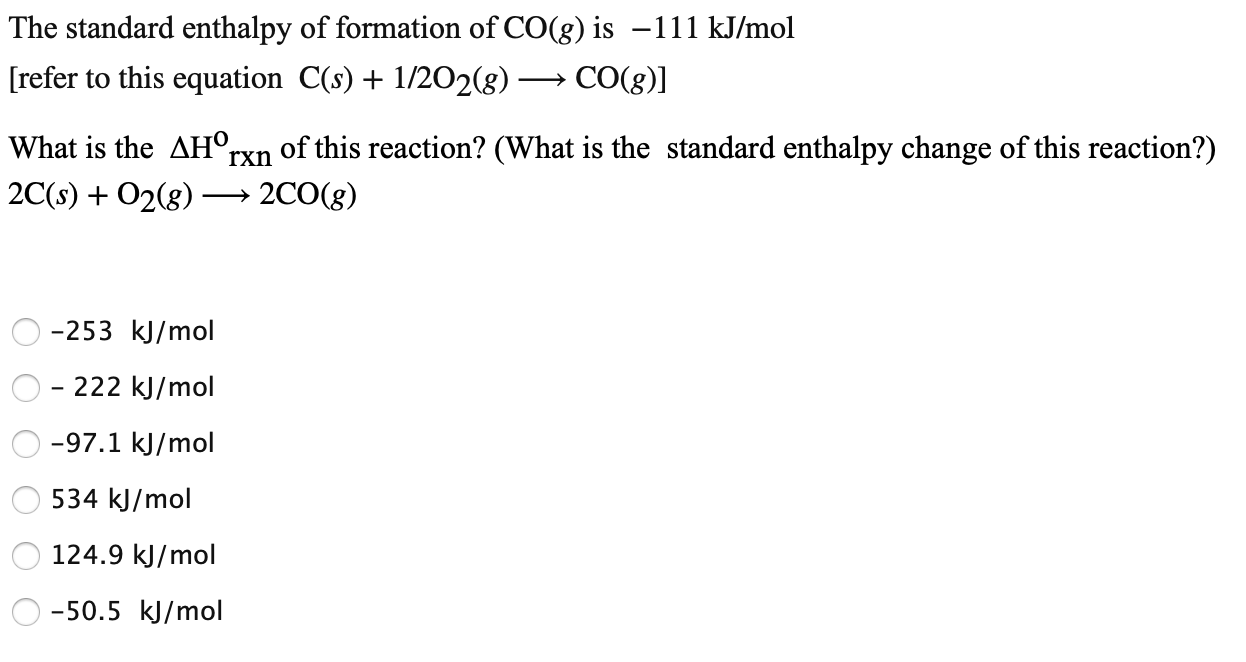
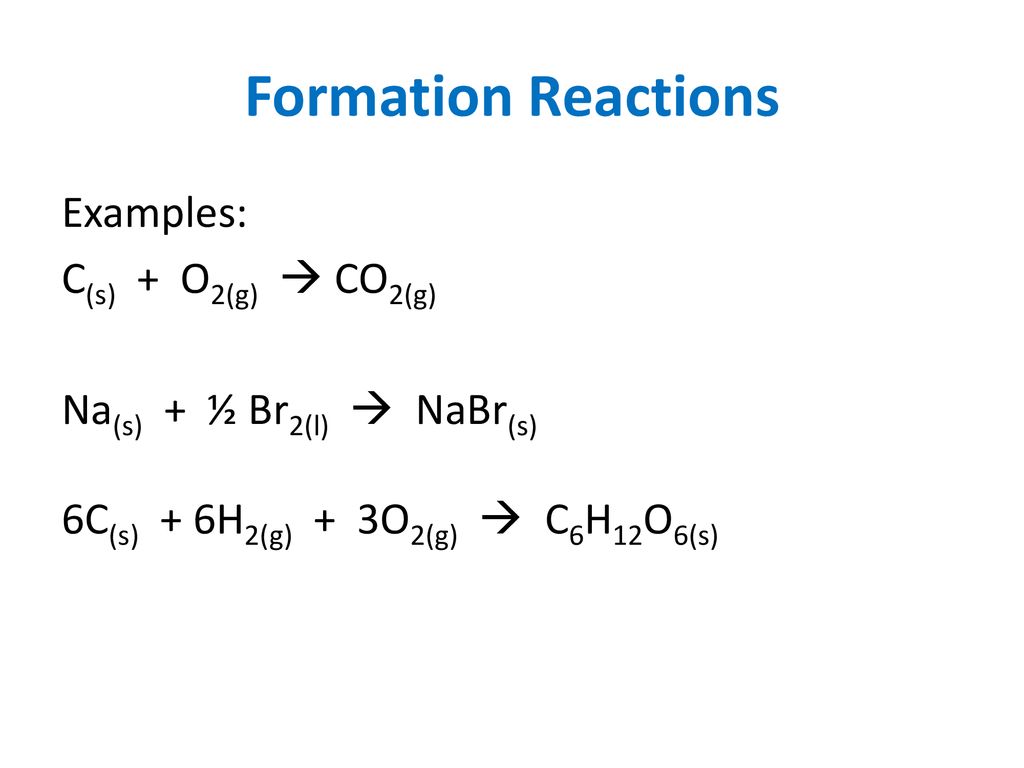
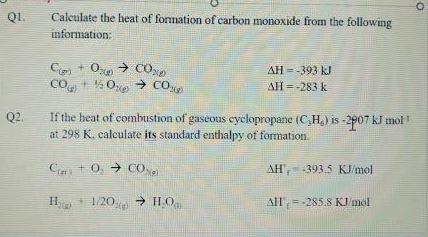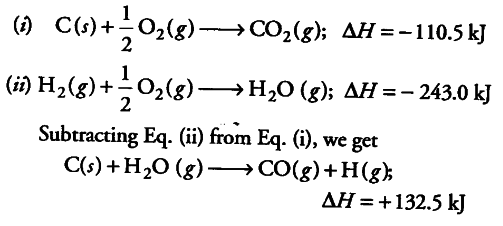The enthalpy of combustion of carbon and carbon monoxide are - 393.5 and - 283 kJ/mol respectively. The enthalpy of formation of carbon monoxide per mole is:
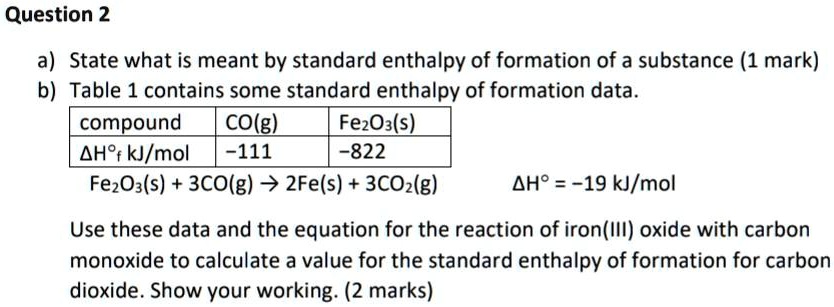
SOLVED: Question 2 a) State what is meant by standard enthalpy of formation of a substance (1 mark) b) Table 1 contains some standard enthalpy of formation data compound COlg) FezOxls) AH'

The heat of combustion of graphite and carbon monoxide respectively are 393.5 kJ mol^(-1) and 283 kJ mol^(-1). Thus, heat of formation of carbon monoxide in kJ mol^(-1) is
The enthalpies of combustion of carbon and carbon monoxide are -393.5 and –283kJ mol^–1 respectively. - Sarthaks eConnect | Largest Online Education Community

Enthalpy of combustion of carbon to CO2 is - 393.5 KJ/mole. The heat released upon the formation of 35.2g of CO2 from carbon and dioxygen gas is.

The enthalpies of combustion of carbon and carbon monoxide are `-390 kJ mol^(-1)` and `-278 kJ mo - YouTube
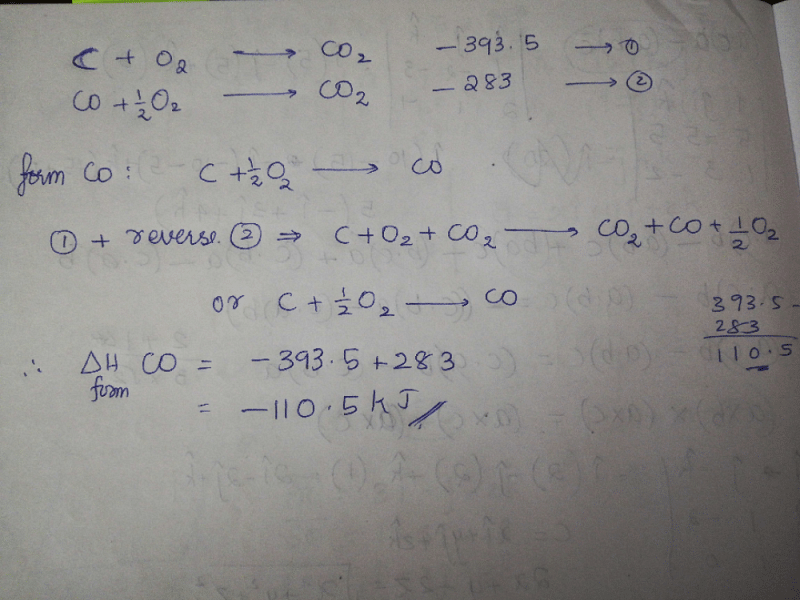
The enthalpies of combustion of carbon and carbon monoxide are -393.5 and -283 KJ mol¹ respectively .The enthalpy of formation of carbon monoxide per mol isa)-110.5 KJb)-676.5 KJc)+676.5 KJd)110.5 KJCorrect answer is
62.The Enthalpies of combustion of carbon and carbon monoxide are 390 kJ and 278kJ respectively. The enthalpy of formation of carbon monooxide is? a) 669 kJ b) 112 kJ c) 112 kJ d) 668 kJ
What is the enthalpy of formation of carbon monoxide in KJ/mol ? C(s) + O2(g) --> CO2(g) ΔH° = -393 kJ 2CO(g) + O2(g) --> 2CO(g) ΔH° = -588 kJ | Socratic

The heat of formations of CO(g) and CO2 (g) are - 26.4 kcal and - 94.0 kcal receptively, The heat of combination of carbon monoxide will be

The enthalpies of formation of CO and `CO_(2)` are `-110.5 KJ mol^(-1)` and `-393.5 KJ mol^(-1)` - YouTube