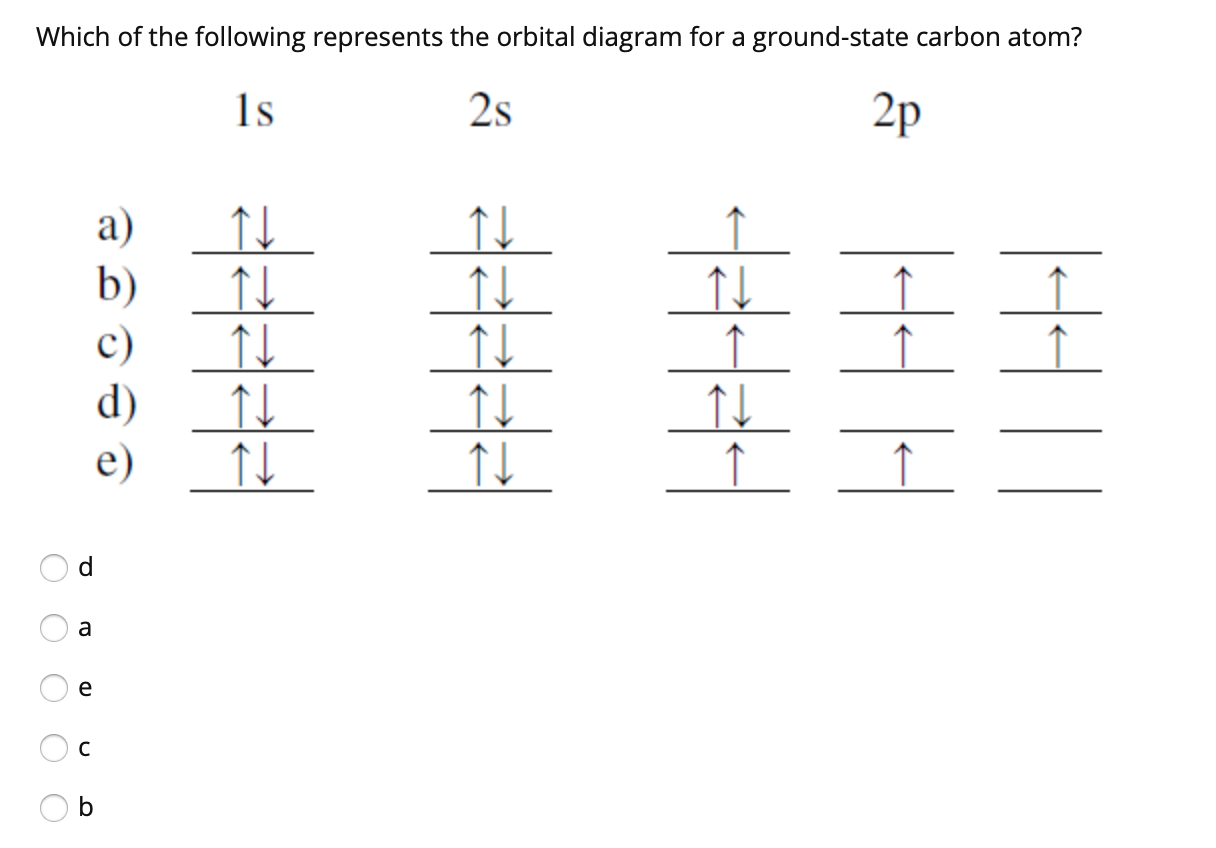
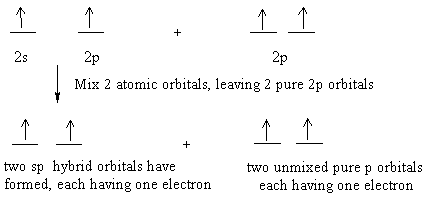
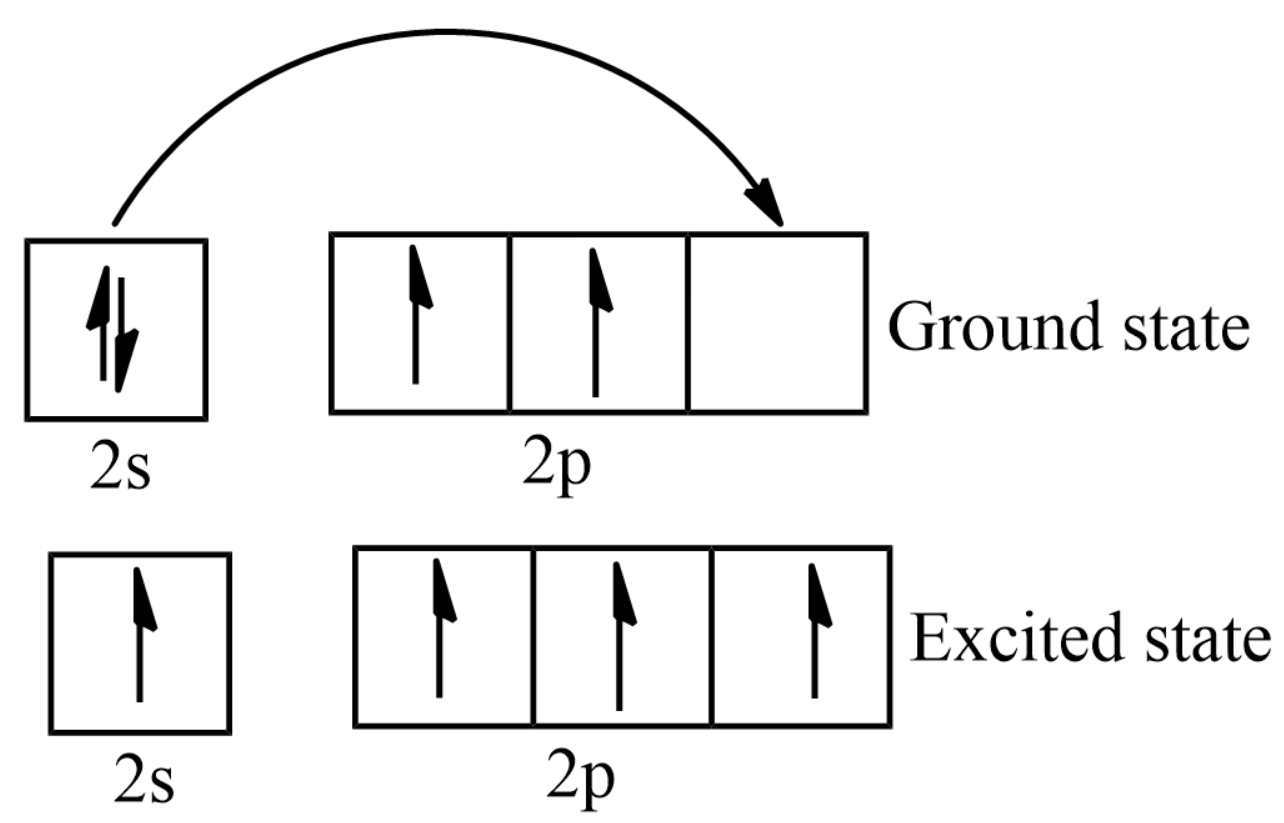
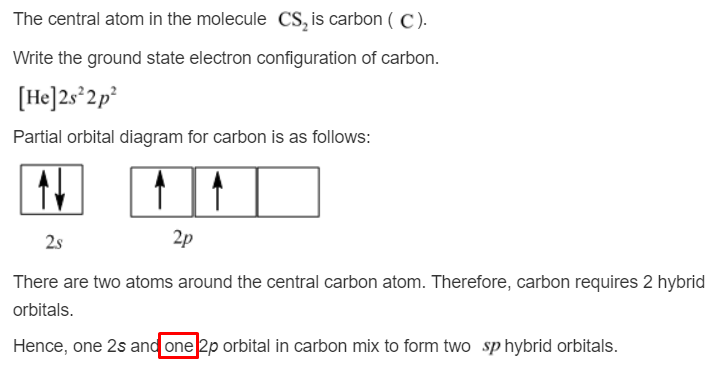
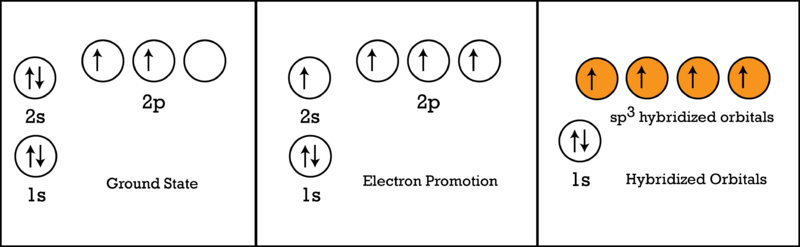
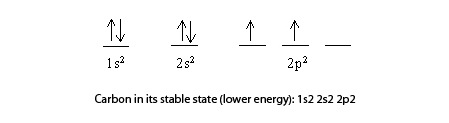
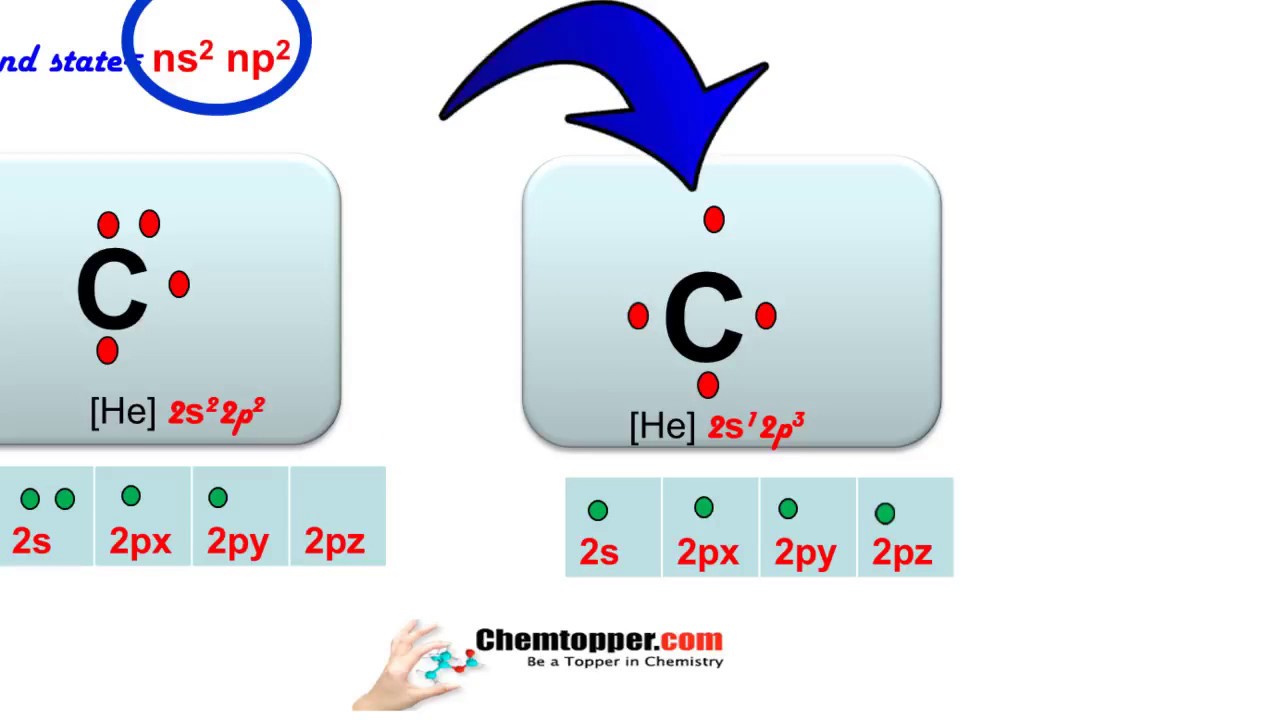
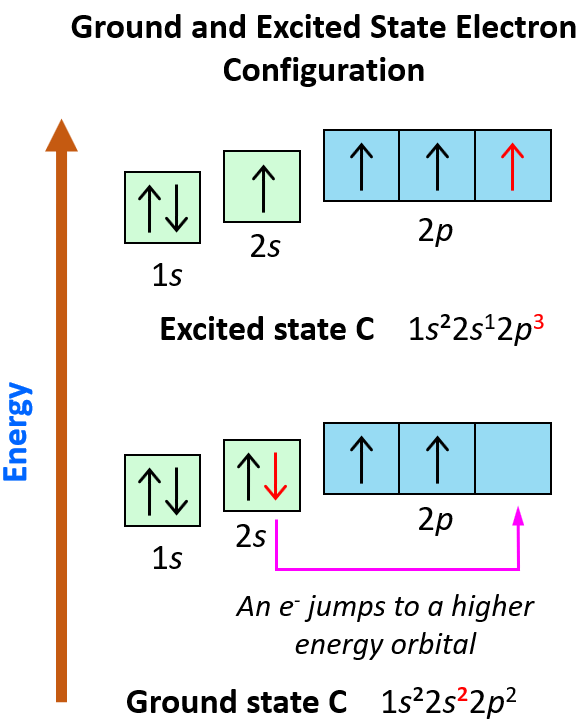
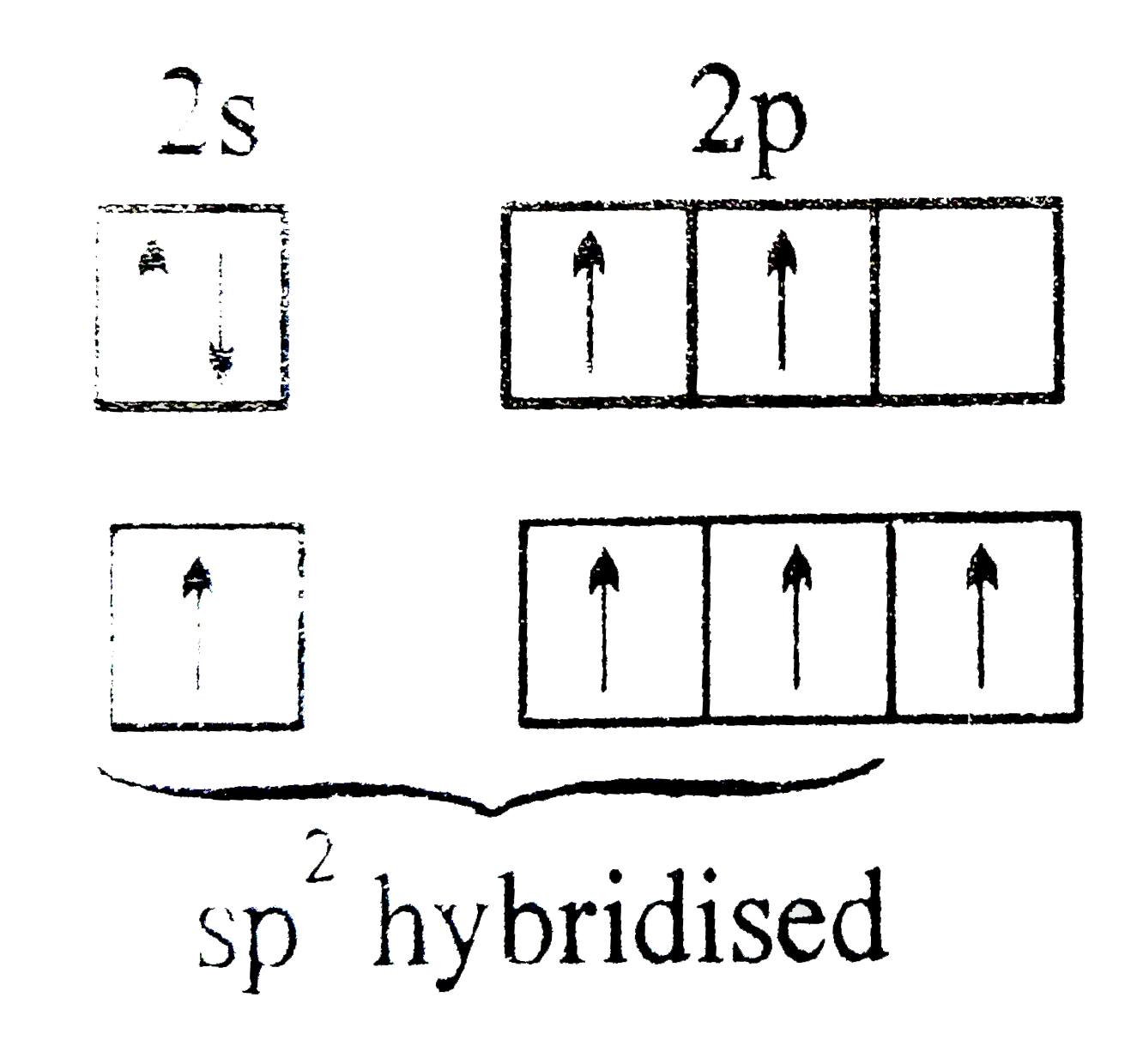Explain the four unpaired electrons in carbon atom through excited state. - Sarthaks eConnect | Largest Online Education Community

Electronic configuration diagram vs energy for carbon atom in its (a)... | Download Scientific Diagram

The study of Carbon. Carbon is in all living things. Carbon is an extremely versatile elements and can bond with other carbon atom to make chains, - ppt download
3: Electronic ground state (a), excited state (b) of the carbon atom,... | Download Scientific Diagram
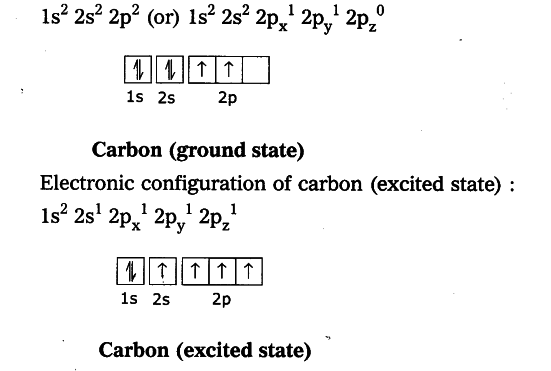
Explain the four unpaired electrons in carbon atom through excited state - CBSE Class 10 Science - Learn CBSE Forum

Explain the following with proper reasoning.Carbon atom is tetrtavalent inspite of the fact that there are only two unpaired electrons in it.
![SOLVED: An excited state of carbon has configuration (1s)2(2s)?(2p) (3p). Determine all of the possible quantum numbers L, S and J and express these as term symbols. [6] Why are there many SOLVED: An excited state of carbon has configuration (1s)2(2s)?(2p) (3p). Determine all of the possible quantum numbers L, S and J and express these as term symbols. [6] Why are there many](https://cdn.numerade.com/ask_images/5f60bdb7ceb741969d3120da01b77069.jpg)
SOLVED: An excited state of carbon has configuration (1s)2(2s)?(2p) (3p). Determine all of the possible quantum numbers L, S and J and express these as term symbols. [6] Why are there many