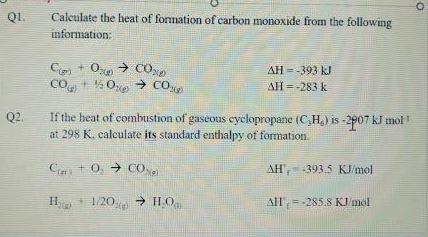Enthalpy of combustion of carbon to CO2 is - 393.5 KJ/mole. The heat released upon the formation of 35.2g of CO2 from carbon and dioxygen gas is.

the heat of combustion of carbon to co2 is -393.5 kj/mol. the heat released upon formation of 35.2 g of co2 - Brainly.in

Question Video: Determining the Standard Enthalpy of Formation of Ethanol Using Standard Enthalpies of Combustion | Nagwa

The heat of combustion of carbon to carbon dioxide is –393.5 kJ/mol. The heat released upon formation of 35.2 g of carbon dioxide from carbon and oxygen gas is :

The heat of combustion of carbon to carbon dioxide is –393.5 kJ/mol. The heat released upon formation of 35.2 g of carbon dioxide from carbon and oxygen gas is :

Enthalpy of combustion of alcohols data trend graph explaining trend pattern determining delta H combustion comparison with ethers equations advanced A level organic chemistry revision notes doc brown

Enthalpy of combustion of carbon to `CO_(2)` is `-393.5 kJ mol^(-1)`. Calculate the heat release... - YouTube

Question Video: Calculating Standard Enthalpy of Combustion of Methane Using Standard Enthalpies of Formation of Methane and Carbon Dioxide | Nagwa

SOLVED: The standard heat of combustion of liquid benzene (giving water vapor) is 40.145kJ/g at 25 0C. The heats of formation of carbon dioxide and water vapor are 393.509 kJ/mol and 241.818

The heats of combustion of carbon and carbon monoxide are 393.5 and 283.5 kJ mol 1 respectively. The heat of formation in kJ of carbon monoxide per mole is:A. 676.5B. 676.5C. 110.5D. 110.5

Enthalpy of combustion of carbon to CO2 is -393.5KJ mol-1. Calculate the heat released upon..... - YouTube

If the heat of combustion of carbon monoxide at constant volume and at 17^o C is - 283.3 kJ, then its enthalpy of combustion at constant pressure( R = 8.314J degree^-1 mol^- )

Ethylene on combustion gives carbon dioxide and water. Its heat of combustion is 1410.0 kJ . mol^-1 . If the heat of formation of CO2 and H2O are 393.3 kJ and 286.2

The heat of combustion of carbon to CO2 is - 393.5 kJ/mol. What is the heat released upon formation of 35.2 g of CO2 from carbon and oxygen gas?

Enthalpy of combustion of carbon to CO 2 is 393.5 kJ mol 1. Calculate the heat released upon formation of 35.2 g of CO 2 from carbon and dioxygen gas.

Calculate the standard heat of formation of carbon disulphide (l). Given that the standard heats of - YouTube

The heat of formation of CO2 is - 94.0 Kcal. What would be the quantity of heat liberated, when 3 g of graphite is burnt in excess of oxygen: -