Question Video: Determining the Products Formed from the Reaction between Sodium Carbonate and Hydrochloric Acid | Nagwa

SOLVED: Hydrochloric acid (HCl) reacts with sodium carbonate (Na2CO3), forming sodium chloride (NaCl), water (H2O), and carbon dioxide (CO2). This equation is balanced as written: 2HCl(aq)+Na2CO3(aq)→2NaCl(aq)+H2O(l)+CO2(g) Part A What volume of 1.75
24. What is the gram equivalent mass of Na2CO3 in a) Na2CO3 + 2HCl= 2NaCl +H2O b) Na2CO3 + HCl = Nacl + NaHCO3

Solved! How many liters of 0.53 M HCl is required to neutralize 0.78 g of sodium carbonate (Na2CO3)? (MM of Na2CO3 = 105. 99 g/mol) 𝟐𝑯𝑪𝒍 + 𝑵𝒂𝟐𝑪𝑶𝟑 → 𝟐𝑵𝒂𝑪𝒍 +
What will be the mass of sodium chloride formed when 5.3 g of sodium carbonate is dissolved in 250 ml of a half molar HCl solution? - Quora

OneClass: 2HCl + Na2CO3 --> 2NaCl + H2O + CO2 If the complete reaction with 1.4252 (plus or minus ...
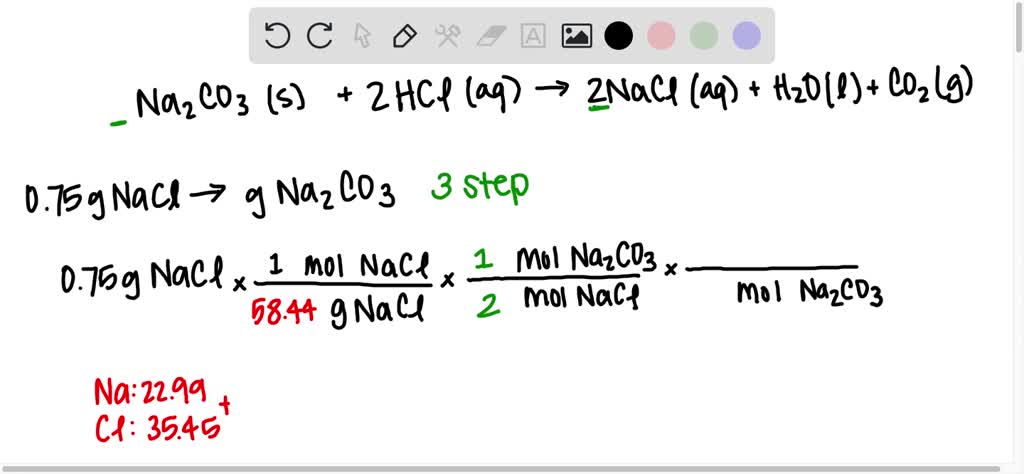
SOLVED: Na2CO3(s) + 2HCl(aq) =2NaCl(aq) + H2O(l)+CO2(g) Using the balanced chemical reaction from step 1, calculate the mass of sodium carbonate ( Na2CO3) that would be required to make 0.75 g sodium chloride.