
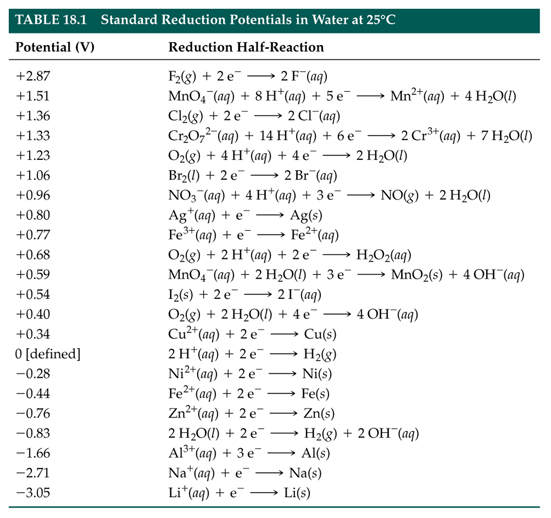
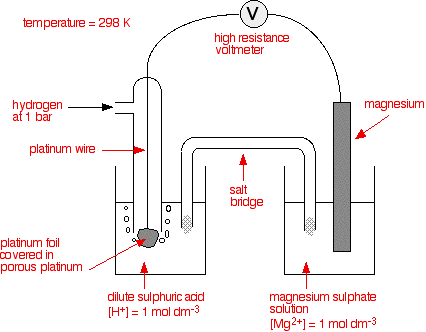
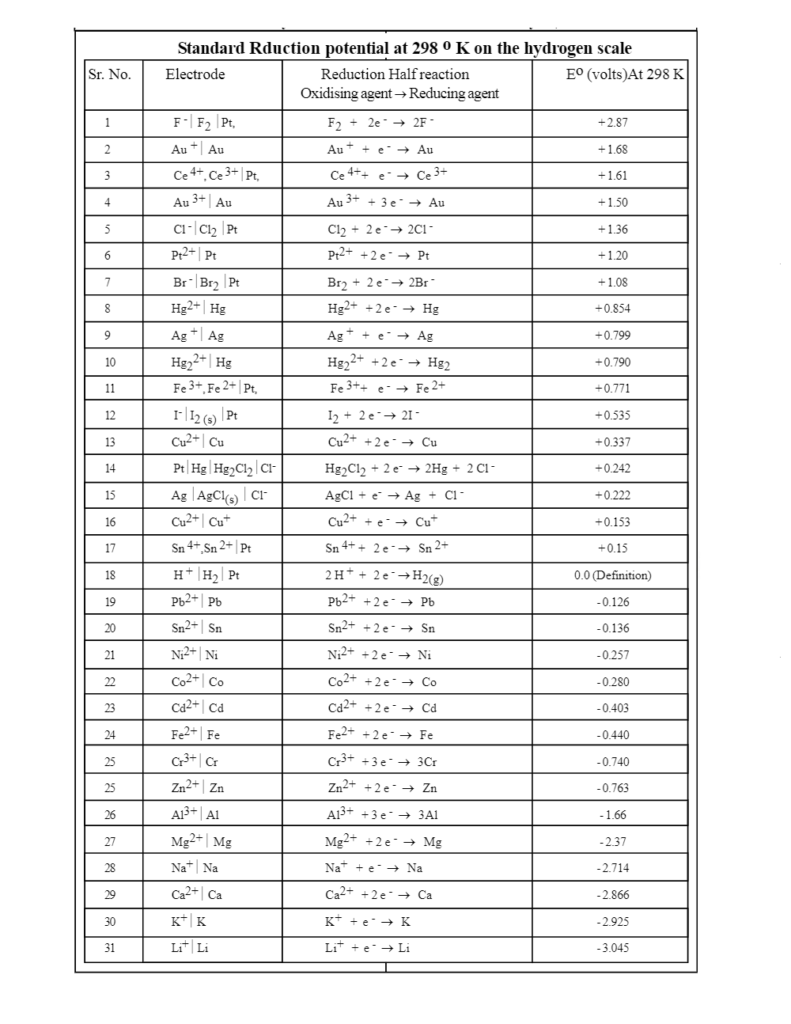
StuDents Oon strike. - ELECTROCHEMICAL SERIES AND ITS APPLICATION:- A list of elements arranged in order on the basis of their standard reduction potential or oxidation potential is called electrochemical series. EXPLAINATION:-

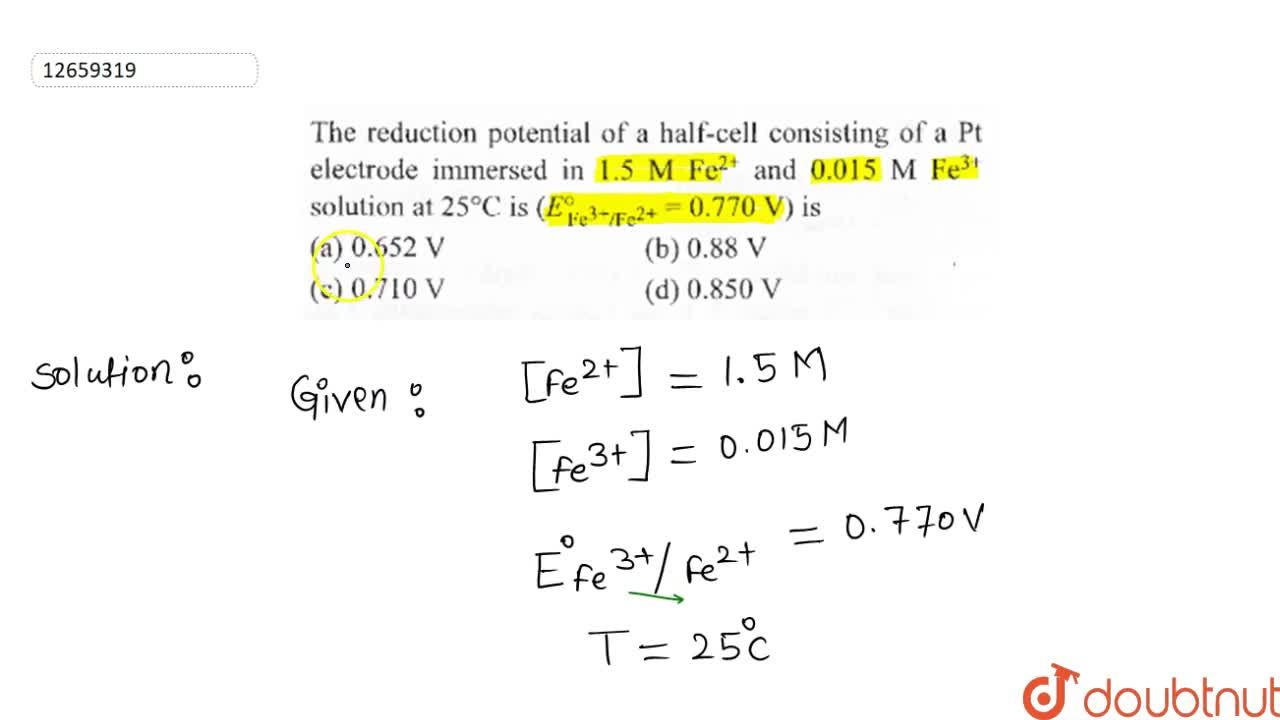
Calculate the reduction potential of a half cell consisting of a platinum electrode immersed in 2.0 MFe^2 and 0.02 M FE^3 solution. Given E^0Fe^3/Fe^+2 = 0.771 V .

Calculate the reduction potential of a half cell consisting of a platinum electrode immersed in 2.0 MFe^2 and 0.02 M FE^3 solution. Given E^0Fe^3/Fe^+2 = 0.771 V .

Applicability of Platinum as a Counter-Electrode Material in Electrocatalysis Research | ACS Catalysis
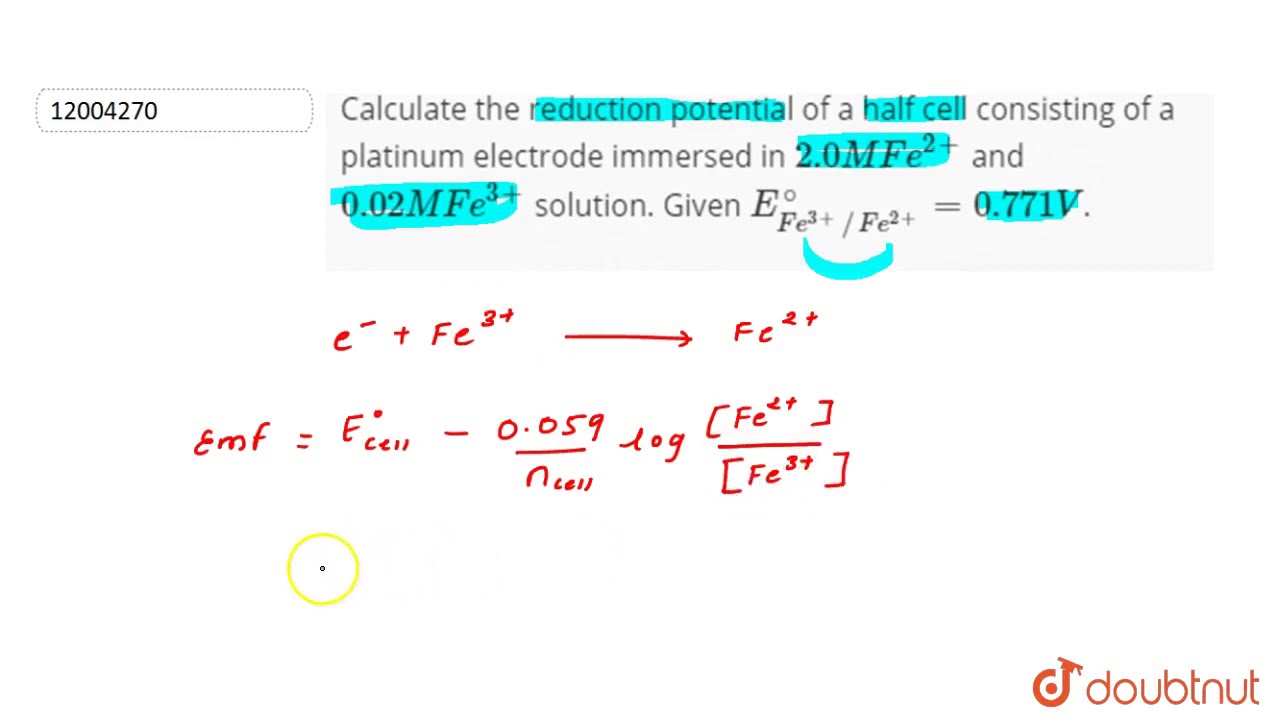
Calculate the reduction potential of a half cell consisting of a platinum electrode immersed in - YouTube

Table 3 from Platinum(II) metal complexes as potential anti-Trypanosoma cruzi agents. | Semantic Scholar

Standard Reduction Potentials for Oxygen and Carbon Dioxide Couples in Acetonitrile and N,N-Dimethylformamide | Inorganic Chemistry

Effect of hydrophobic cations on the oxygen reduction reaction on single‒crystal platinum electrodes | Nature Communications

A-level Chemistry AQA Notes: 3.1.11 Electrode potentials and electrochemical cells (A-Level) - A-LEVEL NOTES

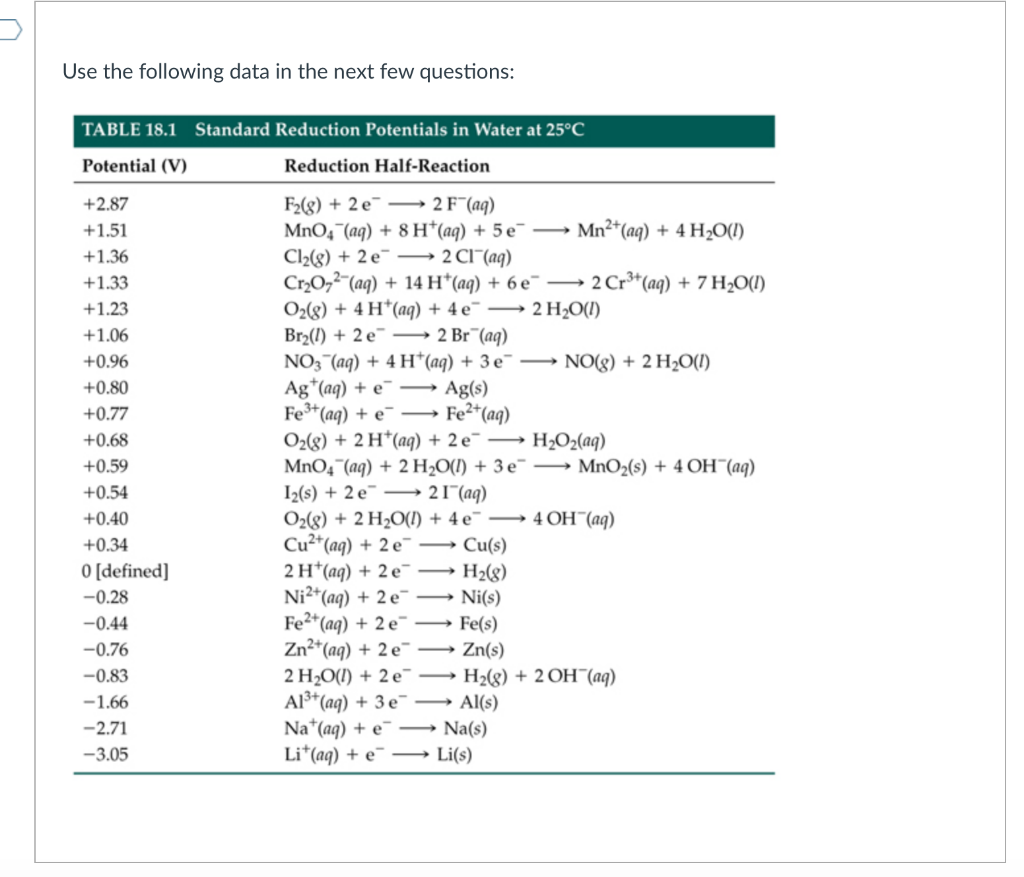


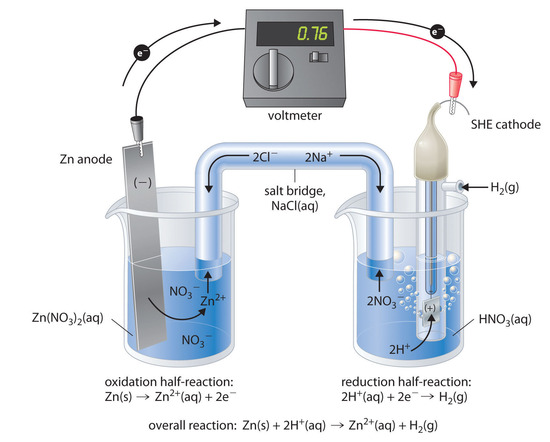




![Standard reduction potentials at 298°K. [24] | Download Table Standard reduction potentials at 298°K. [24] | Download Table](https://www.researchgate.net/publication/316026333/figure/tbl2/AS:650784626708491@1532170554986/Standard-reduction-potentials-at-298K-24.png)

![Electrode potentials [SubsTech] Electrode potentials [SubsTech]](https://www.substech.com/dokuwiki/lib/exe/fetch.php?w=&h=&cache=cache&media=standard_electrode_potential.png)