Morphology of the platinum electrodes used in this study. Sputtered... | Download Scientific Diagram

Applicability of Platinum as a Counter-Electrode Material in Electrocatalysis Research | ACS Catalysis

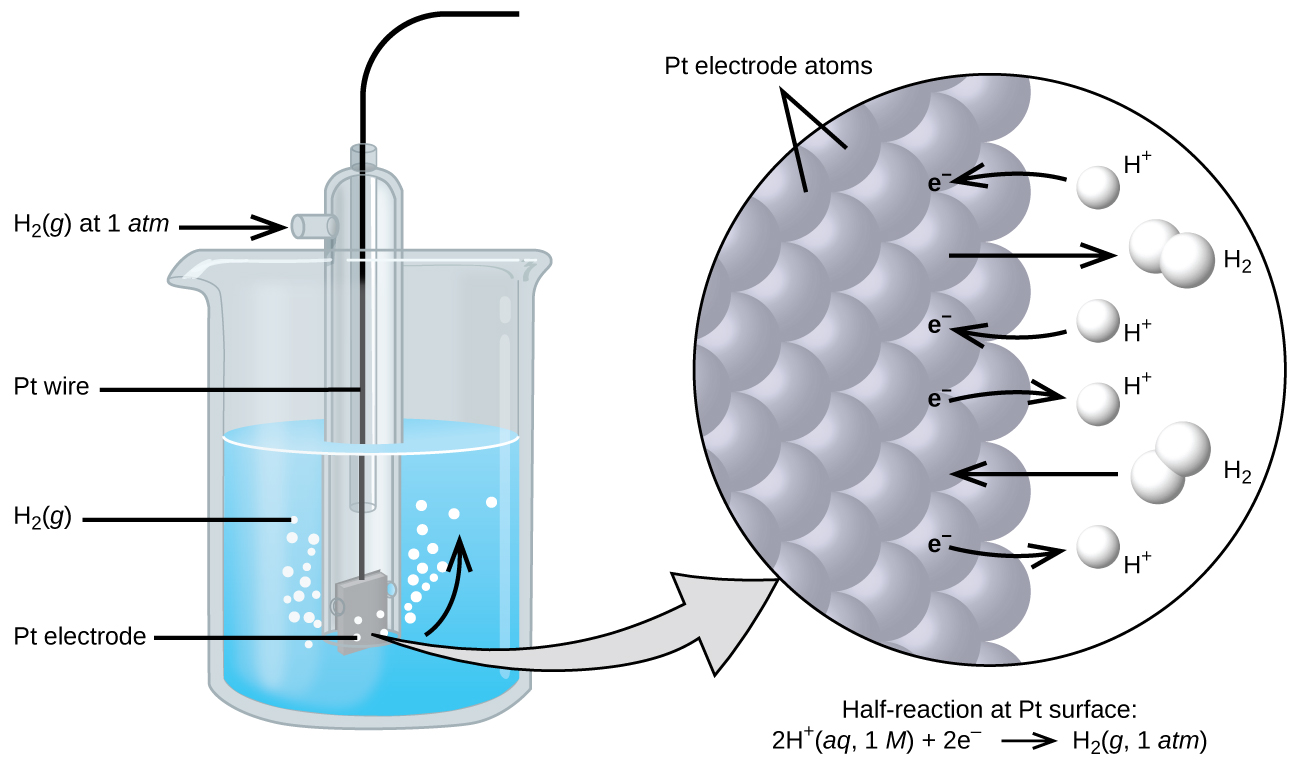
Measuring the Standard Electrode Potential (5.3.4) | CIE A Level Chemistry Revision Notes 2022 | Save My Exams

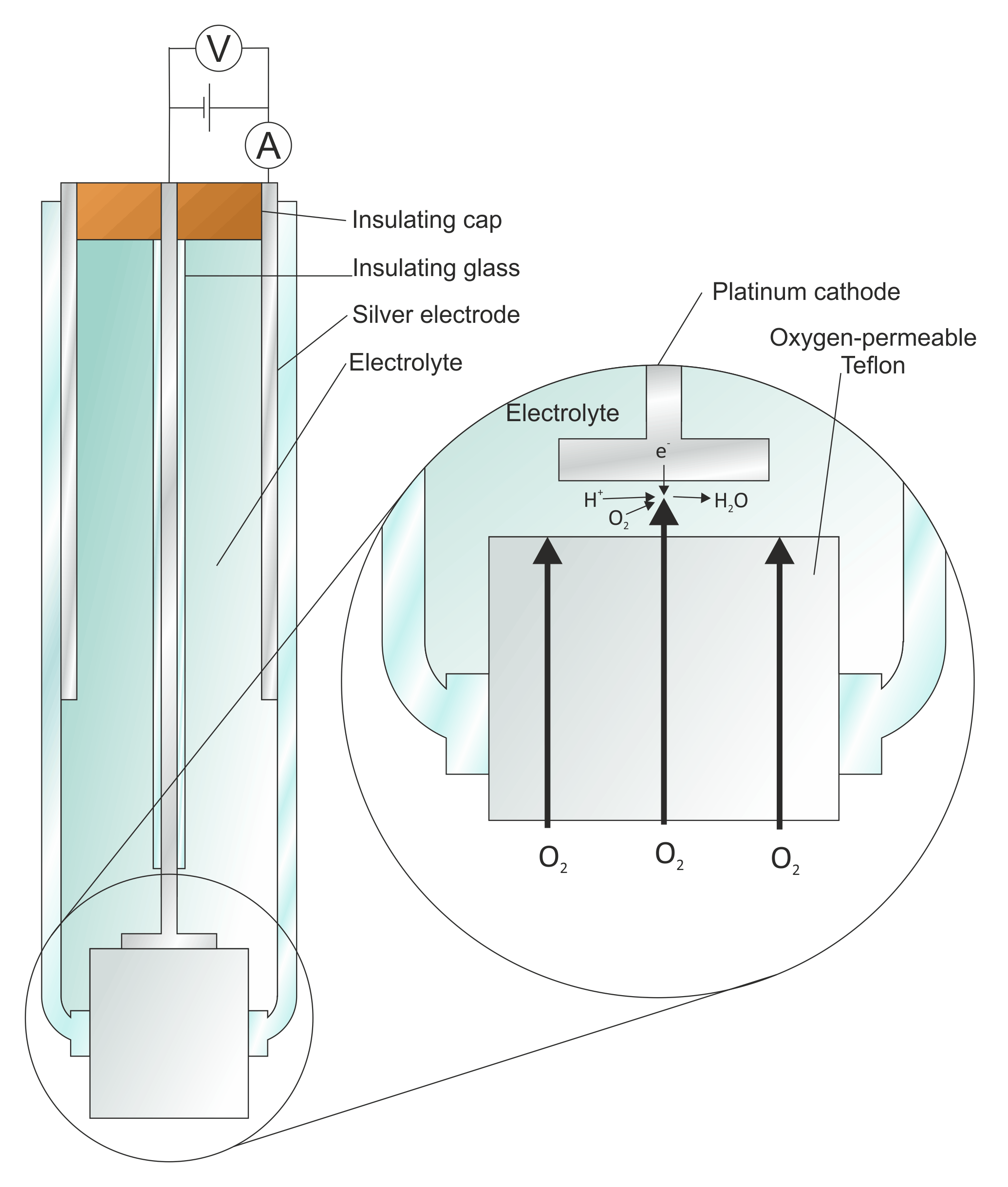
Electrochemical and biological performance of hierarchical platinum-iridium electrodes structured by a femtosecond laser | Microsystems & Nanoengineering

Copper sulphate solution is electrolysed between two platinum electrodes. A current is passed until 1.6 g of oxygen is liberated at anode. The amount of copper deposited at the cathode during the
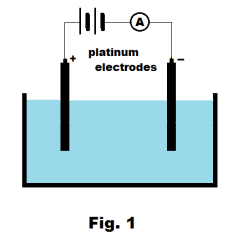
Electrochemistry. Ion. Electrolyte. Electrolysis. Electrolytic cell. Anode. Cathode. Ionic compound. Electroplating. Faraday�s laws of electrolysis. Electrode potential. Voltaic cell. Dry cell. Daniell cell. Gravity cell. Lead storage battery ...

Question Video: Identifying a Major Disadvantage of Using Platinum Electrodes in Electrolysis Experiments | Nagwa
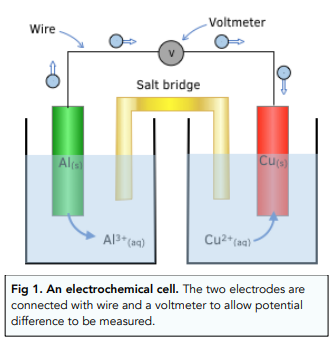
Electrode Potentials and Electrochemical Cells - Representing Electrochemical Cells (A-Level Chemistry) - Study Mind